Abstract
Determining the BUD is very important for ensuring the effectiveness of the compounded nonsterile preparations (CNSPs) and patient safety. Water activity (aw) plays a significant role in pharmaceutical compounding as it directly affects the stability of the CNSPs. Water activity and water content are not the same measurements. Water activity evaluates the energy status of water content in the products. According to USP 975, aw is defined as “A measure of the fraction of total water that is unbound and freely available to participate in chemical, biochemical, or physicochemical reactions or provide an environment that can support microbial growth”. In general, CNSPs with an aw ≥ 0.6 are considered aqueous dosage forms while those with aw < 0.6 are considered nonaqueous dosage forms. If there is no USP−NF monograph or specific stability information available for a specific CNSP, the BUD can be assigned according to the current USP 795 Standard, which is based on aw measurements. The purpose of this short review article is to provide a better understanding of aw and its importance in the determination of the BUD of CNSPs. ‘It mainly focuses on the relationship between water activity and the chemical
degradation as well as microbiological stability of CNSPs.
Keywords: Water activity, moisture content, beyond-use date, degradation, pharmaceutical compounding, Hydrolysis, and microbiological stability.
Introduction
Water, which is utilized as a significant component for a multitude of purposes in the pharmaceutical industry, plays a crucial role in the processing, formulating, and manufacturing of pharmaceutical products, active ingredients, intermediates, and analytical reagents. In addition, water can also be utilized for sanitation purposes. Various grades of water can be attained in the pharmaceutical industry and guidelines may be found in USP monographs regarding information on its use, preparation methodology, and quality requirements.1 Purified water, as defined by the USP monograph, is specifically used as an additive in the production of non-injectable preparations in addition to cleaning non-injectable components and equipment when applied to the matter of the pharmaceutical industry. It can be obtained through a suitable process and must adhere to specific standards set by regulatory bodies like the U.S. Environmental Protection Agency. Purified Water should not contain any additional substances.2
The stability of CNSPs can be affected by each ingredient, including water present in the formula of the CNSPs. When formulating CNSPs, it is essential to use purified water or higher-quality water, if required. This important ingredient can also present concerns about the chemical and microbiological stability of the CNSPs. Pharmacists should ensure the following five types of stability of the compounded preparations: Chemical, Physical, Microbiological, Therapeutic, and Toxicological, as discussed in the USP chapter 1191.3 The amount of water present in the CNSPs can have significant impacts on the above five types of stability of pharmaceutical products.
Water Activity and Stability of CNSPs
Hydrolysis is one of the common forms of a chemical reaction where water is used to break down the chemical bonds in chemical compounds. If the drug substance contains esters, β-lactams, and amide bonds, it may hydrolyze in the presence of water.3 Many drug substances contain water within their crystal lattice (e.g., as hydrate salts).4 Water is also used in the processing, formulating, and manufacturing of pharmaceutical products. Some of the water may be tightly bound and may not be available to participate in chemical reactions, whereas some of the water may be more freely available to participate in reactions such as hydrolysis or may provide an environment that can support microbiological growth. The bacterial cell can only transfer nutrients in and waste materials out through the cell wall.5 The materials, therefore, must be in soluble form to permeate the cell wall. Microorganisms require water to carry out their metabolic activities, and the level of water activity directly affects microbial growth.6
The quality control testing mainly evaluates the total water/moisture content. Total water content is an important quality attribute, and several methods are available to determine water content. Karl Fischer Titration and Loss on Drying are widely used test methods to determine the water content of a sample. Water content in pharmaceutical preparations and water activity are not the same. While water content detects the amount of water within a sample, water activity evaluates the energy status of water content in the products.
US FDA defined aw as “The water activity (aw) of a food is the ratio between the vapor pressure of the food itself, when in a completely undisturbed balance with the surrounding air media, and the vapor pressure of distilled water under identical conditions”.5 The aw increases with the increase in temperature. It is represented on a scale from 0 to 1, where 0 refers to completely dry conditions and 1 refers to pure water. The aw = 0.60 means the vapor pressure is 60 percent of that of pure water.
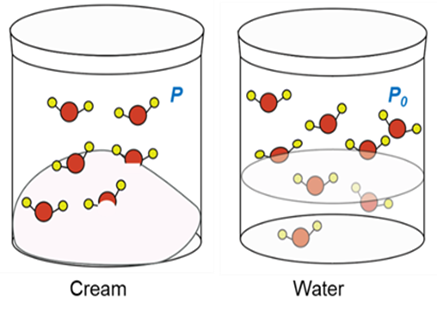
Fig. 1: Water activity (aw) [https://aqualab.com/]
aw = (P/P0)
P = partial vapor pressure of water in the sample
Po = saturation vapor pressure of water
Usually, low aw is used to control microbial decomposition of foodstuffs. Because it makes the products self-preserved. Similarly, low aw is used in pharmaceuticals to control degradation and microbial growth within pharmaceutical drug products. The aw influences the stability of compounded preparations. For example, if the aw of a CNSP is too high, it can promote chemical degradation, microbial growth, and the loss of potency. It is very important to evaluate the stability of CNSPs. Different water activity methods are used to measure the aw in a pharmaceutical product.7 Even though it’s a very simple test, compounding pharmacies are not required to measure aw for CNSPs. We purchased a water activity meter from an online retailer and tested aw of some aqueous compounded preparations such as: O/W Cream (aw =0.89), 30% Poloxamer 407 Gel (aw =0.89), and 2% HPMC suspending vehicle (aw =0.96), and white petrolatum (aw =0.40).
Water Activity and Establishing a BUD for a CNSP
Evaluating water activity helps determine the susceptibility of CNSPs to drug degradation and microbial contamination, as well as assists in determining the BUD. The definitions of aqueous and nonaqueous dosage forms are based on the aw of the aqueous and nonaqueous dosage forms described in USP 795/Table 3.
- Nonaqueous dosage forms with water activity, aw < 0.6 do not support spore germination or microbial growth. Nonaqueous dosage forms fall into two categories: “nonaqueous oral liquid” and “other nonaqueous dosage forms,” such as “capsules, tablets, granules, powders, nonaqueous topicals, suppositories, and troches or lozenges”.
- Aqueous preparations, such as emulsions, gels, creams, solutions, sprays, or suspensions, have an aw ≥ 0.6 and, if required, may contain antimicrobial agents to prevent bacterial, yeast, and mold proliferation.
The revised USP Chapter 795, provides guidelines for CNSPs and their beyond-use date (BUD).8 According to USP guidelines, in the absence of a USP-NF monograph or stability information for CNSP, the BUD can be assigned as follows, if packaged in tight, light-resistant containers at the Controlled Room Temperature or refrigerator:
- Non-preserved aqueous dosage form with aw ≥ 0.6: 14 days when stored in the refrigerator.
- Preserved aqueous dosage form with aw ≥ 0.6: 35 days.
- Nonaqueous oral liquid dosage form with aw < 0.6: 90 days.
- Other nonaqueous dosage forms with aw < 0.6: 180 days.
Conclusion
In summary, water activity is an important factor, as it can help in making decisions to optimize formulations, and make them less susceptible to hydrolysis. Compounders must understand this concept and apply it in assigning BUDs according to USP <975> standards. This review article discusses solely the water activity concept. When determining BUD, compounding personnel should also consider some other factors such as any known instability of API and products.
Acknowledgments
The author is thankful to Dr. Md. Mazharul Islam Chowdhury, Postdoctoral Faculty in the Department of Pharmaceutical Sciences at Appalachian College of Pharmacy for drawing Figure 1.
Reference
- United States Pharmacopeial Convention, Inc. General Chapter: <1231> WATER FOR PHARMACEUTICAL PURPOSES. Accessed February 7, 2024. [Link]
- United States Pharmacopeial Convention, Inc. USP Monographs: Purified Water. Accessed February 7, 2024. [Link]
- United States Pharmacopeial Convention, Inc. General Chapters: <1191> STABILITY CONSIDERATIONS IN DISPENSING PRACTICE. Accessed February 8, 2024. [Link]
- Gupta D, Bhatia D, Dave V, Sutariya V, Varghese Gupta S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Mol J Synth Chem Nat Prod Chem. 2018;23(7):1719. [PubMed]
- Affairs O of R. FDA. Water Activity (aw) in Foods. Accessed February 8, 2024. [Link]
- Cundell T. The role of water activity in the microbial stability of non-sterile pharmaceutical drug products. Eur Pharm Rev. 2015;20(1):58-63. [Link]
- United States Pharmacopeial Convention, Inc. General Chapter〈1112〉 Application of Water Activity Determination to Nonsterile Pharmaceutical Products. [Link]
- United States Pharmacopeial Convention, Inc. General Chapter <795> Pharmaceutical Compounding – Nonsterile Preparations. Accessed February 7, 2024. [Link]
Understanding Water Activity: A Key Component in Determining Beyond Use Date (BUD) in Pharmaceutical Compounding © by Mohammad Faisal Hossain is licensed under CC BY 4.0
Note
Copyright © 2024 Hossain. Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.
