Abstract
Compounding medication plays a crucial role in meeting the specific needs of patients, especially in the pediatric and elderly populations. This article explores the utilization of nanosuspension technology to improve the solubility of BCS Class II drug substances with poor solubility and high permeability characteristics. Through solid dispersion and a quality-by-design (QbD) approach, a stable oral nanosuspension of sildenafil citrate was successfully compounded. The formulation process involved dispersing the drug in Poloxamer 188, and compounding the suspension in a vehicle optimized by Box-Behnken design using HPMC 15 cps, CMC Sodium, and citric acid as independent variables, and viscosity (cp) and zeta potential (mv) as responses. FTIR and DSC analysis revealed the absence of interactions with the formulation components, and the particle size of the dispersed phase (< 1000 nm), viscosity (464 cp), and zeta potential (-37 mv) fell within the desired ranges. Moreover, the polydispersity index (PDI) of the optimized formulations were below 0.7 which also demonstrates acceptability. The F3 and F4 formulations also demonstrated ~ 97% assay by HPLC retention over 14 days at room temperature. This innovative approach offers a promising avenue for 503B compounding pharmacies, allowing for the creation of powdered nanosuspensions that pharmacies can reconstitute while following US FDA guidelines for compounding and dispensing. Introducing nanosuspension technology into compounding practices gives pharmacists a personalized and efficient method for preparing medications, ultimately enhancing patient care and treatment outcomes. Keywords: Pharmaceutical compounding, nanosuspension, solubility, 503A, and 503B Pharmacies
Introduction
Recent advancements in drug delivery systems have revolutionized medication efficacy and availability, offering innovative solutions for addressing various therapeutic challenges.1 Among these advancements, nanosuspensions have emerged as a cutting-edge technology with significant advantages.2 These colloidal dispersions are complex mixtures with drug particles dispersed at the submicron level, signified by their finely dispersed, biphasic composition. This composition features solid drug particles that are smaller than 1 μm.3 Nanosuspensions offer improved stability, solubility, and bioavailability compared to traditional suspensions. Thus, providing a promising solution for overcoming inherent challenges in drug formulations.4 This technology shows significant progress in developing medications that fall under the BCS Class II category, known for their low solubility in water.5 By reducing particle size to the nanoscale, these formulations enhance drug dissolution rates and absorption, potentially resulting in a quicker onset of action and improved therapeutic outcomes.6
Compounding in pharmacy plays a crucial role in personalized medicine by allowing healthcare professionals to create medications that meet the exact needs of individual patients. This personalized approach is essential for enhancing treatment outcomes by developing medications tailored to each patient’s requirements.7 In this study, we are introducing nanosuspension as an innovative compounding method that offers pharmacists an effective way to create personalized liquid medications. This study focuses on Sildenafil as a model drug, which is a poorly soluble compound with high permeability, classified as BCS Class II.8 The objective of this study is to develop a nanosuspension through a solid dispersion technique using the Quality by Design (QbD) approach.9 By analyzing current research and development, we aim to show how this innovative method is creating new opportunities in personalized medicine, leading to customized treatment solutions. This approach could revolutionize pharmacy compounding, offering new possibilities and improving patient care.
Materials
Chemicals and Reagents
The active pharmaceutical ingredient (API) Sildenafil Citrate was obtained as a sample from SMS Lifesciences India Limited, India. Poloxamer 188 and Poloxamer 407, used as hydrophilic carriers, were sourced from BASF, Germany. Methanol, utilized as a solvent for the solid dispersion, was collected from Sabic, Saudi Arabia, while methylene chloride was obtained from Ineor Chlor Ltd, UK. The viscosity-imparting agent, HPMC 15 cps, was acquired from Colorcon, India. Additionally, CMC Sodium, used as a suspending agent, was supplied by Ashland Specialty Ingredients, USA. Citric Acid Monohydrate and Sodium Citrate, both used as buffering agents, were procured from Merck, Germany.
Equipment
The equipment used includes an Ultrasonic Bath (model: Ultrachem M2) from Sonoswiss, Switzerland; a Magnetic Stirrer (model: LMS-1003) from Labtech, Korea; an HPLC (model: Prominence) from Shimadzu, Japan; a Zeta Sizer (model: ZS90) from Malvern, Switzerland; a DSC (model: DSC 4000) from PerkinElmer, USA; a Vortex Mixer (model: VM 2000) from Digisystem Laboratory Instruments Inc, Taiwan; and a Vacuum Oven (model: UFP 400) from Memmert, Germany. Additionally, a Rotary Evaporator (model: R-100) from Buchi, Switzerland, and an FTIR (models: Prestige-21 and IR Tracer-100) from Shimadzu, Japan, were also utilized.
Methods
Compounding suspension methods cover a wide range of techniques used in pharmacy settings, from traditional mortar and pestle methods to advanced equipment like homogenizers and sonicators.10 Pharmacists skillfully combine commercial products and raw materials to create customized suspensions, tailoring formulations to meet individual patient needs and preferences.11 Nanosuspensions, a cutting-edge development, are typically generated through two primary approaches—top-down and bottom-up methodologies.12 The top-down strategy involves reducing the size of large drug particles through processes like milling or high-pressure homogenization, while the bottom-up approach entails dissolving the drug in an organic solvent and precipitating it with an anti-solvent in the presence of a stabilizer.5 There are advantages and disadvantages in both approaches, and these techniques are mainly used for pharmaceutical manufacturing on the largest scale of production.13 However, after a thorough review, we could not find any articles of interest related to the compound. It is noteworthy that a standardized compounding method for nanosuspensions is currently unavailable, indicating a need for innovative approaches in this field. In our study, we are using the method developed by Rahman et al., which entails solid dispersion followed by dispersing granules in a suspending vehicle-optimized by the QbD approach. This presents a unique and promising technique in this emerging field.9
Compounding nanosuspension:
- Dissolve the API in the hydrophilic excipient using the organic solvent.
- Remove the solvent by using an evaporator.
- Sieve the solid dispersion through a mesh screen to achieve the desired particle size.
- Dry the sieved material overnight at 60°C in a vacuum oven.
- Disperse the final solid dispersion powder in water to produce highly concentrated nanosuspension.
- Incorporate the nanosuspension into a suitable suspending vehicle to create the final stable suspension.
Formulation Development
Poloxamer 188 and Poloxamer 407 were selected for preparing the Sildenafil Citrate (SLC) nanosuspension. Initial solid dispersions (SD) were formulated using a 1:2 ratio of SLC: Poloxamer 188 (F1) and SLC: Poloxamer 407 (F2). The API-excipient mixtures were dissolved in a 10% w/w methanol: Dichloromethane (50:50 ratio) solvent. Solid dispersions were then prepared using the solvent evaporation method with a rotary solvent evaporator. The resultant SDs were crushed using a mortar and pestle, followed by sieving through a 0.60 mm (#30 mesh) screen to obtain uniform particles. The SD preparations were dried at 60°C in a vacuum oven overnight. DSC characterization revealed that the API did not convert into an amorphous form. Subsequent dispersion of the SD powders in water by mild sonication showed that SLC: Poloxamer 188 SD dispersed easily, while SLC: Poloxamer 407 did not disperse and instead produced a sticky non-uniform slurry. Therefore, Poloxamer 407 was discontinued from further studies. To address this issue, further trials were conducted using 1:3 (F3) and 1:4 ratios of SLC: Poloxamer 188 ( F4). DSC studies (Figure 1) confirmed the presence of amorphous API in both cases, leading to the production of nanosuspensions upon dispersion in water. A 20% w/w dispersion was made in water with SD powder in each case.
Additionally, API-excipient compatibility was evaluated through FTIR and DSC analysis of API and excipients individually and as a 1:1 binary mixture, revealing no interactions. The resultant nanosuspensions were further characterized for PSD, polydispersity index (PDI), and zeta potential. While PSD and PDI confirmed nanosuspension production for F3 and F4,22 as the PSD was less than 1000nm and the PDI was less than 0.7, instability was observed in the nanosuspensions based on the zeta potential (ZP) data,23 as the ZP was less than -30mv (Table 1).
Table 1: PSD, PDI & ZP for the Solid dispersion (SD) formulation with different ratios of API and polymers.
| Formula | Sildenafil Citrate | Poloxamer 188 | Poloxamer 407 | PSD | PDI | ZP | ||||||
| F1 | 1 | 2 | – | 2607 | 2498 | 2558 | 1.000 | 1.000 | 1.000 | 0.138 | -0.227 | 0.327 |
| F2 | 1 | – | 2 | 3223 | 3307 | 3180 | 1.000 | 1.000 | 1.000 | -0.072 | -0.209 | 0.012 |
| F3 | 1 | 3 | – | 385.0 | 321.9 | 330.1 | 0.412 | 0.339 | 0.407 | 0.097 | 0.075 | -0.059 |
| F4 | 1 | 4 | – | 429.8 | 458.0 | 466.0 | 0.341 | 0.390 | 0.411 | 0.032 | -0.006 | -0.352 |
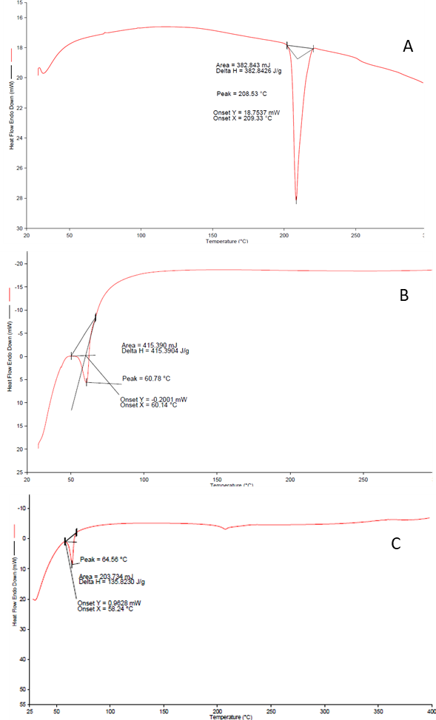
Figure 1. DSC curve of A. sildenafil citrate B. Poloxamar 188 and C. Mixture (Poloxamar 188 & API)
Optimization of suspending vehicle:
The response surface methodology (RSM) is applied for the design and development of various pharmaceutical formulations because it involves the least number of experiments. Therefore, it is faster and more economical than the traditional approach of formulation development of dosage forms.14 Box-Behnken designs (BBD) and central composite designs (CCD) are two major experimental designs used in RSM. 3 factors and 3 levels of BBD were applied in the current study, as BBD is most effective in the case of such designs.15
One of the major stability problems of the nanosuspension is aggregation of particles, resulting in hard cake formation. Yield stress can be introduced in the suspension by incorporating a network structure in the continuous phase. Such suspensions remain immobile unless the applied shear crosses a specific value. The suspensions, therefore, show kinetic stability where particles remain immobile and dispersed within the network. By gelling the continuous phase, with the help of appropriate excipients, this kind of structure can be achieved.16 Examples of such agents are Alginates, HPMC, Xanthan Gum, CMC Sodium, etc. These excipients prevent the particles of nanosuspension from contact by introducing stereospecific blockade between them. Nanosuspensions particles and inhibits the particles from contacting. The increase in stability can be confirmed by measuring the zeta potential.24,25 However, zeta potential can also be modified by adding Citric Acid and Sodium Citrate in the preparations to increase the suspension stability.17
Table 2: The optimized suspension vehicle was obtained using the Box-Behnken DOE model. Independent variables for designing of suspension vehicle:
| Independent variables | Level | ||
| -1 | 0 | +1 | |
| HPMC 15 cps | 0.2 | 0.6 | 1 |
| CMC Sodium | 0.1 | 0.375 | 0.65 |
| Citric Acid | 0.1 | 0.2125 | 0.325 |
Table 3: Designed runs using Box-Behnken design and experimental observation of responses for stabilization of prepared nanosuspensions:
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | |
| Run | A:HPMC | B:CMC Na | C:Citric Acid | Viscosity | Zeta potential |
| g | g | g | cp | mv | |
| 1 | 1 | 0.1 | 0.2125 | 138 | 18 |
| 2 | 1 | 0.375 | 0.1 | 147 | 10 |
| 3 | 0.6 | 0.65 | 0.325 | 90 | 28 |
| 4 | 0.6 | 0.375 | 0.2125 | 86 | 19 |
| 5 | 0.2 | 0.375 | 0.325 | 43 | 25 |
| 6 | 0.2 | 0.1 | 0.2125 | 39 | 17 |
| 7 | 0.2 | 0.375 | 0.1 | 40 | 5 |
| 8 | 0.6 | 0.1 | 0.325 | 84 | 30 |
| 9 | 1 | 0.375 | 0.325 | 156 | 40 |
| 10 | 0.6 | 0.1 | 0.1 | 79 | 4 |
| 11 | 0.2 | 0.65 | 0.2125 | 44 | 18 |
| 12 | 1 | 0.65 | 0.2125 | 149 | 23 |
| 13 | 0.6 | 0.65 | 0.1 | 80 | 8 |
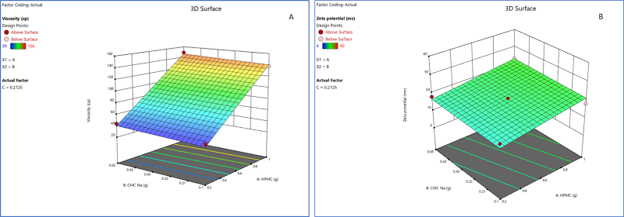
Figure 2. (A) 3D response surface plot for Response 1 (viscosity) for optimization of suspension vehicle; and (B) 3D response surface plot for Response 2 (zeta potential) for optimization of suspension vehicle.
Analysis of Response 1 (viscosity, cp):
Response 1 followed a linear model with an adjusted R2 value of 0.9736 and a predicted R2 value of 0.9571. The amount of HPMC 15 cps (factor A) played a significant role in Response 1 (figure contour chart and 3D surface plot). The model F-value of 148.28 implies the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise. P-values less than 0.0500 indicate model terms are significant.
Analysis of Response 2 (zeta potential, mv):
Response 2 followed a linear model with an adjusted R2 value of 0.9202 and a predicted R2 value of 0.8680. The amount of HPMC 15 cps (factor A) and citric acid monohydrate (factor C) played a significant role in Response 2 (figure contour chart and 3D surface plot). The model F-value of 47.14 implies the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise. P-values less than 0.0500 indicate model terms are significant.
To optimize the suspension vehicle, constraints were set first (Table 4). Among 70 solutions found from the software, the one with a maximum desirability of 0.904 was selected as the optimized solution; consisting of HPMC 15 cps 1 g, CMC Sodium 0.650 g, and Citric Acid Monohydrate 0.325 g. The predicted viscosity and zeta potentials were 149.634 cps and -35.096 mv, respectively.
Table 4: Table for constraints
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
| A:HPMC | is in range | 0.2 | 1 | 1 | 1 | 3 |
| B:CMC Na | is in range | 0.1 | 0.65 | 1 | 1 | 3 |
| C:Citric Acid | is in range | 0.1 | 0.325 | 1 | 1 | 3 |
| Viscosity | maximize | 39 | 156 | 1 | 1 | 3 |
| Zeta potential | maximize | 4 | 40 | 1 | 1 | 3 |
Results and Discussion
After optimizing the methods for the vehicle formulation, the suspending vehicle was prepared by dispersing HPMC 15 cps (1 g), CMC Sodium (0.650 g), and Citric Acid Monohydrate (0.325 g) in purified water. To adjust the pH to 5, Sodium Citrate (0.691 g) was added, and the final volume was made up to 100 ml with purified water. The viscosity of the optimized suspending vehicle was found to be 464 cP using 30 rpm, with a Zeta Potential (ZP) ranging from -37.1 to -38.4. F3 and F4 SDs were dispersed in water with the help of mild sonication to produce nanosuspension. Then these nanosuspensions were incorporated into the suspending vehicle. Each 5 ml of the final suspension contained 71 mg of Sildenafil Citrate, equivalent to 50 mg of Sildenafil. The final formulations were characterized as shown in Table 5.
Table 5: Viscosity and zeta potential values of the final formulations.
| Formula | Zeta potential (mv) | Viscosity (cp) | ||
| F3 | -36.5 | -34.9 | -34.1 | 423 |
| F4 | -35.3 | -37.0 | -35.9 | 377 |
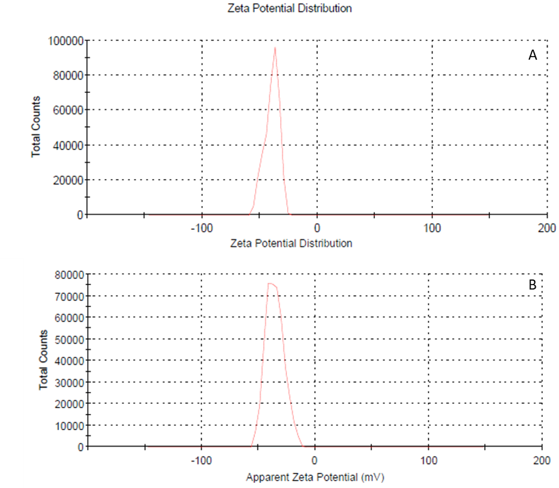
Figure 3. Zeta potential distribution of the final formulations A. F3 and B. F4 by Zetasizer, Malvern Instruments
The stability of the formulations was assessed over 14 days, and the formulations were found to be chemically stable in terms of purity (% assay) over the two weeks. (Non-preserved aqueous dosage form with aw ≥ 0.6: 14 days when stored in the refrigerator.18,19 The drug content was determined using HPLC and is presented in Figure 4. Additionally, the solid dispersion powder was analyzed by Gas Chromatography (GC 2010-plus, Shimadzu, Japan) to ensure that residual solvents (Methanol: 1305 ppm (Spec: NMT 3000 ppm) and Dichloromethane: 39 ppm (Spec: NMT 600 ppm) were within the specifications. The methanol and dichloromethane levels were found to be well below the accepted maximum limit.20
Table 6: Stability Study reports (% Assay by HPLC) of F3 & F4 formulations.
| Formulation | Assay (Day 0) | Assay (Day 14) |
| F3 | 98% | 97% |
| F4 | 97% | 97% |
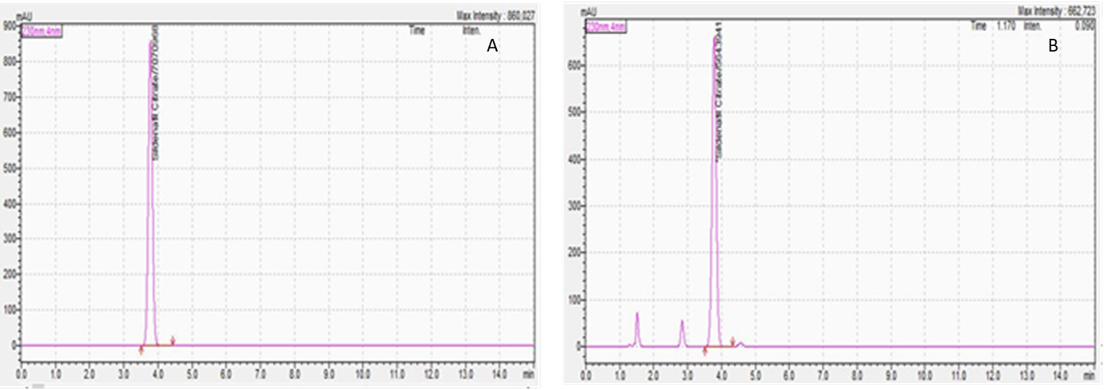
Figure 4. Representative high-performance liquid chromatogram of the A. Standard, and B. Test sample. RT of SL is 3.7 minutes.
Section 503A and section 503B of the Federal Food, Drug, and Cosmetic Act pertain to human drug compounding.21 The limitations of a 503A facility can present challenges when compounding suspension products due to the research required. On the other hand, a 503B outsourcing facility is equipped to compound large quantities with proper quality control and assurance. These outsourcing facilities can conduct research and develop solid dispersion and suspending vehicles that 503A pharmacies can utilize. The prepared blends can then be used by 503A pharmacies to dispense nanosuspensions through simple reconstitution methods. Introducing nanosuspensions as an innovative compounding method provides pharmacists with a personalized approach to medication creation, ultimately enhancing patient care and treatment outcomes. Nanosuspensions offer various advantages, including improved drug solubility, enhanced stability, bioavailability, and uniformity of preparation.
Pharmacists can determine the equivalent dose of a nanosuspension that may enhance drug bioavailability by using the formula F1 x Dose1 = F2 x Dose2, where F1 represents the bioavailability factor of the commercial oral dose form, and F2 represents the bioavailability factor of the nanosuspension. (REF) The assessment of the nanosuspension’s bioavailability should be conducted by the 503B pharmacy. This calculation assists pharmacists in determining the appropriate equivalent dose for the nanosuspension based on its enhanced bioavailability potential.
While compounding suspensions may involve higher initial development costs, the long-term benefits include cost savings, reduced drug wastage, and the production of more uniform medications. Ultimately, compounding suspensions can be valuable for pharmacy settings seeking to optimize drug formulations for improved patient outcomes.
Conclusion
In conclusion, nanosuspension technology offers a promising solution for overcoming solubility challenges associated with BCS Class II drugs. By reducing particle size and employing advanced techniques such as solid dispersion and a quality-by-design approach, pharmacists can improve drug solubility, which may lead to increased bioavailability and efficacy.
For 503B pharmacies, it’s crucial to accurately calculate equivalent doses when developing nanosuspension products, as differences in drug availability compared to FDA-approved products may arise. The enhanced solubility and improved drug absorption provided by nanosuspension technology present significant benefits in personalized medication compounding. This advancement highlights a valuable opportunity for enhancing patient care in pharmaceutical practice.
Acknowledgments
The authors are thankful to Incepta Pharmaceuticals, Bangladesh for providing the facility to execute the experiment to complete this research.
Authors’ Contributions
Each author actively engaged in analyzing and discussing the results, thereby contributing to the finalization of the manuscript. SA and MB conducted an in-depth literature review of equal contributions. MRR and MFH provided supervision throughout the article, ensuring a comprehensive interpretation of the findings.
Conflict of interest
The authors declare no conflict of interest.
References
- Ezike TC, Okpala US, Onoja UL, et al. Advances in drug delivery systems, challenges and future directions. Heliyon. 2023;9(6):e17488. doi:10.1016/j.heliyon.2023.e17488
- Chavhan R. NANOSUSPENSIONS: ENHANCING DRUG BIOAVAILABILITY THROUGH NANONIZATION. Ann Pharm Fr. Published online June 28, 2024. doi:10.1016/j.pharma.2024.06.003
- Zhang J, Xie Z, Zhang N, Zhong J. Chapter 13 – Nanosuspension drug delivery system: preparation, characterization, postproduction processing, dosage form, and application. In: Andronescu E, Grumezescu AM, eds. Nanostructures for Drug Delivery. Micro and Nano Technologies. Elsevier; 2017:413-443. doi:10.1016/B978-0-323-46143-6.00013-0
- Patel VR, Agrawal YK. Nanosuspension: An approach to enhance solubility of drugs. J Adv Pharm Technol Res. 2011;2(2):81-87. doi:10.4103/2231-4040.82950
- Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics. 2018;10(3):134. doi:10.3390/pharmaceutics10030134
- Dizaj SM, Vazifehasl Zh, Salatin S, Adibkia Kh, Javadzadeh Y. Nanosizing of drugs: Effect on dissolution rate. Res Pharm Sci. 2015;10(2):95-108.
- Carvalho M, Almeida IF. The Role of Pharmaceutical Compounding in Promoting Medication Adherence. Pharmaceuticals. 2022;15(9):1091. doi:10.3390/ph15091091
- Miranda C, Pérez-Rodríguez Z, Hernández-Armengol R, Quiñones-García Y, Betancourt-Purón T, Cabrera-Pérez MÁ. Biowaiver or Bioequivalence: Ambiguity in Sildenafil Citrate BCS Classification. AAPS PharmSciTech. 2018;19(4):1693-1698. doi:10.1208/s12249-018-0982-7
- Rahman MR, Shill DK, Kumar U, Hossain AMA, Bachar SC, Rouf ASS. Formulation and Evaluation of Ledipasvir Nano-suspension Through QbD Approach. Dhaka Univ J Pharm Sci. 2023;22(2):173-188. doi:10.3329/dujps.v22i2.69324
- Team TBCC. How Compounding Pharmacies Mix Compounds. Accessed July 9, 2024. https://blog.bigcountry.pharmacy/how-compounding-pharmacies-mix-compounds
- PharmaNewsIntelligence. Understanding the Basics of Pharmaceutical Compounding. PharmaNewsIntelligence. Published June 23, 2023. Accessed July 9, 2024. https://pharmanewsintel.com/features/understanding-the-basics-of-pharmaceutical-compounding
- Ahmadi Tehrani A, Omranpoor MM, Vatanara A, Seyedabadi M, Ramezani V. Formation of nanosuspensions in bottom-up approach: theories and optimization. DARU J Pharm Sci. 2019;27(1):451-473. doi:10.1007/s40199-018-00235-2
- Salazar J, Müller RH, Möschwitzer JP. Combinative Particle Size Reduction Technologies for the Production of Drug Nanocrystals. J Pharm. 2014;2014:265754. doi:10.1155/2014/265754
- Malakar J, Sen SO, Nayak AK, Sen KK. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc. 2012;20(4):355-363. doi:10.1016/j.jsps.2012.02.001
- Manohar M, Joseph J, Selvaraj T, Sivakumar D. Application of Box Behnken design to optimize the parameters for turning Inconel 718 using coated carbide tools. In: ; 2013. Accessed July 23, 2024. https://www.semanticscholar.org/paper/Application-of-Box-Behnken-design-to-optimize-the-Manohar-Joseph/71cd6e8b855b79976d9f799c7c06382d76c35e41
- Thursday, December 1, 2016. Controlling the Stability of Medicinal Suspensions. Accessed July 23, 2024. https://www.americanpharmaceuticalreview.com/Featured-Articles/331619-Controlling-the-Stability-of-Medicinal-Suspensions/
- The Role of Citric Acid in the Stabilization of Nanoparticles and Colloidal Particles in the Environment: Measurement of Surface Forces between Hafnium Oxide Surfaces in the Presence of Citric Acid | Langmuir. Accessed July 23, 2024. https://pubs.acs.org/doi/abs/10.1021/acs.langmuir.7b03116
- Hossain MF. Understanding Water Activity: A Key Component in Determining Beyond Use Date (BUD) in Pharmaceutical Compounding. J Pharm Compd Ther. 2024;1(1):1-3.
- USP General Chapter 795 | USP. Accessed July 23, 2024. https://www.usp.org/compounding/general-chapter-795
- Q3C Tables and List Guidance for Industry.
- Research C for DE and. FD&C Act Provisions that Apply to Human Drug Compounding. FDA. Published online August 13, 2021. Accessed July 23, 2024. https://www.fda.gov/drugs/human-drug-compounding/fdc-act-provisions-apply-human-drug-compounding
- Gumustas, M., Sengel-Turk, C. T., Gumustas, A., Ozkan, S. A., & Uslu, B. (2017). Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics.
- Jacobs, C., & Müller, R. H. (2002). Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharmaceutical Research, 19(2).
- Larsson, M., Hill, A., & Duffy, J. (2012). Suspension stability: Why particle size, zeta potential and rheology are important Product Technical Specialists Rheometry Products Malvern Instruments Limited. Annual Transactions of the Nordic Rheology Society, 20(1988).
- Wang, Y., Zheng, Y., Zhang, L., Wang, Q., & Zhang, D. (2013). Stability of nanosuspensions in drug delivery. In Journal of Controlled Release (Vol. 172, Issue 3).
Sildenafil Nanosuspension by QbD: Unlocking New Possibilities in Pharmaceutical Compounding © 2024 by Md. Rezowanur Rahman, Mohammad Faisal Hossain, Sartaz Araf, A.B.M. Asikur Rahman is licensed under CC BY 4.0
Note
Copyright © 2024 Rezowan et al. Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.
