Abstract
Master Formulation Record (MFR) and Compounding Record (CR) must be created for each single formulation of a compounded nonsterile preparation (CNSP). Documentation is one of the most important components of quality assurance to prevent errors and ensure accuracy and safety in the compounding process. The MFR can be duplicated, leaving blank spaces to fill in the details needed to finish the CR. In this article, the MFR and CR for lidocaine topical gel preparation are combined for 503A pharmacies. Most of the information that is required as per the current USP <795> Standard is included. The manual compounding method was validated and characterized using HPLC. The study results indicated that the compounding method produced a uniform, reproducible (%Assay ± SD; 101.6% ± 2.2) and elegant (clear hydro-alcoholic) gel preparation with an excellent yield (~99.0%). This compounding protocol may serve as a valuable resource for compounding professionals to minimize errors and to ensure accuracy, consistency, quality, and safety in the compounding process. Keywords: Lidocaine Topical Gel, Master Formulation and Compounding Records, 503A pharmacies, USP <795>
Introduction
Lidocaine is frequently used as a local anesthetic drug. It is a weak base with a pKa of 7.9 and falls under the BCS class II drug (high permeability and low solubility) category.1 The molecular weight is 234.34 g/mol. Lidocaine Hydrochloride monohydrate (LHM) is the salt form of the Lidocaine and it is a white powder freely soluble in water. The molecular weight of the salt is 288.82.2 LHM salt contains ~81% of the base (Lidocaine). This percentage can be used to calculate the active drug moiety of the drug (to convert the base to salt or salt to the base form of the Lidocaine) during compounding. Patients undergoing specific medical procedures can manage pain by applying lidocaine topical preparations.3,4
Pharmaceutical compounding involves creating specialized drugs to address individual patient requirements. A thorough document that provides detailed directions for compounding a particular medication is called a “Master Formulation and Compounding Records”. USP General Chapter <795> Pharmaceutical compounding—nonsterile preparation defined Master Formulation Record and Compounding Records as “A master formulation record (MFR) is a detailed record of procedures that describes how the CNSP is to be prepared” and “A compounding record (CR) documents the compounding of each CNSP.”5 To ensure accuracy and uniformity in the compounding process, this record is used as a guide for compounders. In this protocol, the master formulation and compounding records are combined to make one protocol. This protocol is developed for topical lidocaine gel (TLG) compounded preparation.
Materials
Pharmaceutical grade drug and excipients, and ACS/HPLC grade chemicals were used to compound and analyze the formulation to evaluate the quality. The items were purchased from commercial suppliers: Lidocaine Hydrochloride, Carbomer 940, Propylene Glycol, Ethyl alcohol, Acetonitrile, Phosphoric acid, Monobasic potassium phosphate, Trifluoroacetic acid. Distilled water was obtained through the in-house water distillation system located at ACP Faculty Lab.
Compounding Methods
The Master Formula was originally developed by the author and published in IJPC.6 In this article, the master formula and compounding method were optimized for manual compounding. According to this method, all formulation materials were to be placed directly into the dispensing container in the proper order while being weighed on the weighing scale. The ingredients were mixed with a glass rod to complete the formulation. The master formula and the detailed compounding procedure are described in Table 1.
Table 1
The Master Formulation and Compounding Records for Lidocaine Topical Gel *Please download the article.
HPLC Analysis
The following chromatographic conditions were used to calculate the content of lidocaine present in the gel. The active ingredient (lidocaine) was extracted using a solution of acetonitrile and 0.1% phosphoric acid (1:1) with sonication for 10 minutes and shaking intermittently. The standard curve of lidocaine was used to calculate the content (Figure 1). The chromatographic separation was achieved by the use of a Shimadzu Prominence HPLC system with a Waters XBridge C-18 (4.6 mm x 100 mm, 10 µm) column using isocratic elution mode with a flow rate of 1 mL/min. The detection wavelength was 220 nm. The mobile phase consisted of acetonitrile and a buffer (25 mM KH2PO4; pH adjusted to 3.6 with trifluoroacetic acid) at a ratio of 20:80 (v/v). The pH of CLG was determined by using the pH test paper.
Results and Discussion
In the formulation, propylene glycol and ethanol are employed as co-solvents to improve medication penetration through the skin from the gel matrix. To decrease potency errors and prevent cross-contamination which produces a decrease in the amount of mass to clean up, a direct compounding process with a cumulative weighing approach was used. Following process and formulation optimization, TLGs were analyzed. The study’s findings show that it is uniform and reproducible (%Assay ± SD; 101.6% ± 2.2). The gel preparation can be made in less than ten minutes by taking advantage of this user-friendly compounding method. The pH is between 5 -6. An approximate loss of 1g of gel on the glass rod may occur, thus the process loss is very minimal.
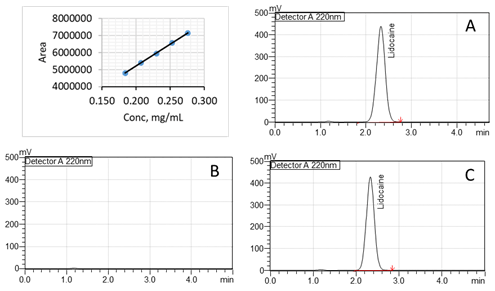
Fig. 1. Linear curve (0.184 mg/mL to 0.276 mg/mL) of lidocaine; B: Representative high-performance liquid chromatogram of the A: Diluent, B: Lidocaine Standard and C: Lidocaine Gel sample (RT of lidocaine is 2.5 minutes).
When the pharmacy receives an Active Drug Substance (API) from a supplier or manufacturer they must verify the test results such as purity (% Assay), Impurities, water content, etc reported on the Certificate of Analysis (CoA) with the specifications published in the USP Monograph for that specific API. They also need to verify the type, whether it is a base (Lidocaine) or the Salt (Lidocaine Hydrochloride). If the pharmacy has the salt form of the lidocaine in stock, then they need to adjust the weight of the API by calculating the conversion factor (CF) using their Molecular Weights (CF=MW of the Lidocaine/MW of the lidocaine Salt = 0.81) to get the equivalent amount of Active drug. For instance, 2 g of Lidocaine is equivalent to 2.5 g of Lidocaine Hydrochloride Monohydrate. Before compounding any preparation, the responsible personnel must check and verify all ingredients’ expiration dates, and the purity (% Assay) of API and write on the MFR & CR. Based on the purity, the compounder may need to adjust the amount of the API required to compound the preparation. For example: as per USP Monograph, Lidocaine should contain NLT 97.5% and NMT 102.5% of lidocaine. So if the purity of the API available in the pharmacy is 97.5% then it may be necessary to adjust the weight using the purity factor. In this situation, the compound should use 2.05 g of Lidocaine instead of 2 g of Lidocaine to compound the gel preparation.
Calculation:
Purity Factor = 97.5/100 = 0.975
Amount of API required to get the correct dose = 2 g/0.975= 2 g/0.975= 2.05 g
The author believes that the inclusion of calculation formulas with appropriate instruction in the compounding protocol will significantly minimize compounding errors. All of the important findings and information obtained from USP, CoA, and Literature Review for every compounded preparation should be included in the MFR and CR.
Conclusions
Simplified compounding approaches and the application of QA and QC in compounding have a significant impact on minimizing errors and improving preparation quality and patient safety. This compounding protocol will serve as a valuable resource for student pharmacists, practicing pharmacists, and compounding professionals to ensure accuracy, consistency, and safety in the compounding process.
Acknowledgments
The author is thankful to Dr. Randy Mullins, Associate Professor of Pharmacy Practice and Department Chair of the Pharmaceutical Sciences Department, and Shivkumar Vijayakumar, Pharm. D. Candidate of 2024 at Appalachian College of Pharmacy for their time and effort in reviewing the manuscript and providing inputs. The author is also thankful to Dr. Chowdhury for helping during the HPLC analysis.
References
- Amorphous solid dispersions of lidocaine and lidocaine HCl produced by ball milling with well-defined RAFT-synthesised methacrylic acid polymers – ScienceDirect. Accessed January 9, 2024. [Link]
- LIDOCAINE HYDROCHLORIDE INJECTION USP. Lake Forest, IL. Hospira, Inc. Accessed January 9, 2024. [Website]
- Mayo Clinic. Lidocaine (Topical Application Route). Accessed January 9, 2024. [Website]
- Lidocaine Hydrochloride Jelly USP, 2%. Lake Forest, IL. Akorn, Inc. Accessed January 9, 2024. [Link]
- United States Pharmacopeial Convention, Inc. General Chapter <795> Pharmaceutical Compounding – Nonsterile Preparations. Accessed January 10, 2024. [Link]
- Hall E, Levesque D, Rashid M, Mullins R, Hossain MF. A Simplified Method for Compounding Lidocaine Topical Gel: A Demonstration of Quality Assurance to Prevent Errors in 503A Pharmacies. Int J Pharm Compd. 2021;25(3):197-204. [PubMed]
Compounding Lidocaine Topical Gel: An Exhibit of a “Master Formulation and Compounding Records” for 503A pharmacies by Mohammad Faisal Hossain is licensed under CC BY 4.0
Note
Copyright © 2024 Hossain. Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.
