Abstract
While extended-release dosage forms are an effective method to increase patient adherence and improve pharmacotherapy, there are still issues with formulation preparations regarding pharmaceutical compounding. Physicians in the United States often prescribe compounded hormone preparations. The compounded preparations may be called Slow Release (SR) capsules, but do we know the exact release kinetics and the quality of the preparations? Formulation development for SR capsule dosage forms requires proper knowledge and training of the compounder and an appropriate facility. The exact release kinetics and the quality of the SR compounded capsules might differ from our expectations due to a change in the excipients and/or their ratio, which can alter the efficacy and lead to inconsistency. In the current 503A pharmacy setting (traditional compounding pharmacy), researching to develop SR formulations is impractical. Considering these issues, we developed the innovative concept that various strengths of drug-excipient combinations for slow-release (SR) preparations could be pre-made, tested, and distributed by a 503B outsourcing facility following the policies of the US FDA. These pre-made blends would then be used by 503A pharmacies to compound SR capsules using either the simple Alligation Method or the direct filling technique with certain limitations. This new idea was validated and confirmed by evaluating the release kinetics of thiamine hydrochloride SR capsules compounded from different strengths of drug-excipient blends prepared by both dry and wet granulations.
Keywords: Extended-release dosage forms, slow-release capsule, HPMC, drug-release kinetics, 503A, and 503B Pharmacies.
Introduction
The extended-release (ER) dosage forms are a notable addition to the pharmacy field for drugs with a very short half-life compared to conventional solid dosage forms. Terms such as “prolonged release,” “delayed release,” “long-acting,” and “sustained release” have been used to describe ER dosages. However, “ER” is the official term used for article title.1 The term slow release is often used to describe or label these formulations. Pharmacists receive frequent requests from prescribers to compound SR capsules. SR becomes the preferred descriptor because its definition is very imprecise and does not have a set release rate/profile connected to it.2 Currently, 503A pharmacy practices for slow-release formulation based on the idea of releasing active medicament over an extended period of time3,4 and the most commonly used excipients for the purpose are hydrophilic controlled-release polymers sourced from cellulose, namely hydroxypropyl methylcellulose (HPMC or Hypromellose) derivatives.4,5 However, the release of active ingredients from a dosage form depends not only on polymer properties but also on the physicochemical properties of the drug itself. In addition, limited opportunities in 503A pharmacies made it more challenging to evaluate and confirm the release kinetics of individual medications for a certain period of time that 503B outsourcing facilities can ensure.
Researchers used the different percentages of Methocel E4M (HPMC 2910) and Methocel K100M (HPMC 2208) in the formulation of SR capsules of different drug molecules such as morphine sulfate, oxycodone, theophylline, niacinamide, and liothyronine sodium.7–11 The actual value of the %drug released of some formulations was not reported, so it was estimated from the %drug released vs. time curve. The results of a study conducted with morphine sulfate SR capsules containing approximately 36% of Methocel K100M (ID#C) without lactose demonstrated that the in vitro drug release was prolonged, and approximately 80% of the drug was released at the five-hour time point.6 Another study conducted on the release kinetics of morphine sulfate and oxycodone hydrochloride SR capsules showed approximately 80% of both drugs were released in 5 hours from the SR capsules containing 40% of Methocel E4M with anhydrous lactose.7 The in vitro dissolution profiles of morphine sulfate from the two different types of HPMC showed a very similar release profile. Another study conducted on the release kinetics of SR capsules showed how dissolution rates vary based on the percentage of polymer used.8 The drug was released at a slower rate from the 60% Methocel E4M compared to the 40% Methocel E4M as it contains more polymer. The 40% Methocel E4M formulation displayed an in vitro release profile similar to the two separate studies reported above. The drugs morphine sulfate, oxycodone hydrochloride, and niacinamide from SR capsules containing 40% Methocel E4M show similar in vitro release kinetics.
Although similar in vitro dissolution profiles were found for the three drugs above from the SR capsules containing 40% Methocel E4M, this does not necessarily correlate with the release kinetics of other drugs. For instance, theophylline and T3 showed different release kinetics from the HPMC polymers. For T3 SR capsules, the release rates of the drug from the capsule compounded with 40% Methocel E4M is also lower than other drugs reported in this study. This study also showed that Methocel E4M has a more consistent drug release profile than Methocel K100M.5 Based on the literature review, not only does the drug release depend upon the polymer used, but the physicochemical properties of the drug itself also play a significant role. The in vitro drug release from 40% HPMC may vary for different drugs. To address this issue, we came up with the idea that different strengths of drug-excipient blends for SR preparations could be premade, tested, and distributed by the 503B outsourcing facility. Then, 503a pharmacies can use the blends to compound SR capsules. We hypothesized that compounding SR capsules using different premix blends prepared by Alligation Calculation would have minimal to no effect on drug release kinetics.
Materials
USP–NF-grade drug substances and excipients were used to compound SR capsules. Thiamine hydrochloride was obtained from ACROS Organics. HPMC was purchased from Spectrum and Anhydrous Lactose was purchased from Medisca. All solvents used were HPLC grade and analytical reagent grade. Methanol and Glacial acetic acid were purchased from Fisher Scientific, and distilled water was obtained from the in-house water distillation apparatus.
Methods
Granulation processes
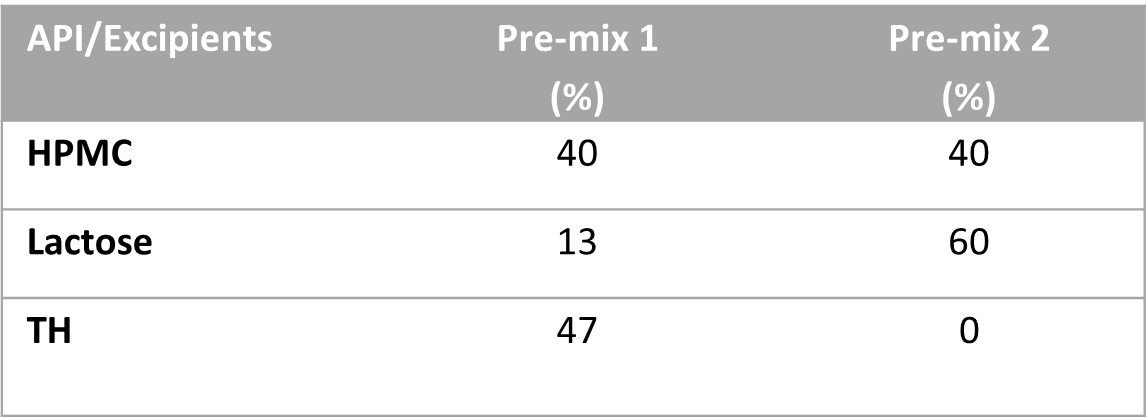
To test this idea, we have used thiamine hydrochloride (TH) as an active pharmaceutical ingredient, HPMC 2910 as a gelling agent, and lactose as a filler. We have made two different blend strengths by both dry mix and wet granulation methods to compound 75 mg and 100 mg of TH SR capsules using the Alligation Method used in pharmaceutical compounding. So, we prepared the following premix blends: Pre-mix 1 (a blend of 40% of HPMC, 13% of Lactose, and 47% TH) and Pre-mix 2 (a blend of 40% of HPMC, 60% of Lactose, and 0% TH). The targeted powder fill volume of each capsule was 425 mg/capsule. Wet granulation was done using 16% purified water by spray method and drying at 52°C for about 25 minutes.
Table 1
Formulations and Alligation Method.
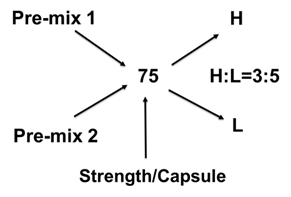
Following the initial manuscript review, the authors implemented a suggestion from one of the reviewers and prepared the Pre-mix 1 blend using the dry granulation method and compounded 100 mg of the SR capsule into a size 00 capsule shell, 50 mg into a size 1 capsule shell, and 25 mg into another size 1 capsule shell using the direct filling technique. Furthermore, a separate batch of 25 mg SR capsules was produced by adding lactose as a filler to adjust the fill volume. The purpose of this study was to investigate the feasibility of directly filling a pre-made blend into different capsule shells to compound various strengths of SR capsules.
HPLC Chromatographic conditions and analysis
The stock solution was prepared by dissolving 25 mg of thiamine hydrochloride in 100 ml water (0.25µg/ml). Calibration standards were prepared from the stock solution by adding the required milliliters of diluents to attain final concentrations of 120,100, 80, 60,40, 20, 10, and 5 µg/mL, respectively. According to USP, a mixture of distilled water, methanol, and Glacial acetic acid (73: 27: 5 v/v) was prepared and used as a mobile phase. The flow rate of the mobile phase was 1 ml/min. An X-bridge C18; 5µm, 4.6 X 150 mm column at room temperature (250 C) in a Shimadzu (Japan) HPLC system was used to quantify TH. Ultraviolet detection was achieved with a SPD-20AC UV-VIS detector at 275 nm (λ max). Figure 3 shows the representative HPLC chromatograms and the linear curve.
Results and Discussion
The release of the active drug from the capsule is dependent on the gelatinous form taken on by HPMC once it is exposed to an aqueous medium. The dissolution rate relies upon the gel’s viscosity and diffusion of the active drug through the gel layer. Properties of certain drugs can cause the gel to have different impacts on drug release. The gel breaks down as water uptake in the gastrointestinal tract increases. This causes the gel to become thinner or diluted, which in turn causes active drugs to be released and then absorbed by the body. Highly soluble drugs often form pores in the gel, leading to increased dissolution rates. Due to the decreased compaction of the materials in the capsules, the gel layer formed within the capsules tends to be weaker than tablets.2 In assessing drug release rates in compounded SR capsules, we conducted a literature review of HPMC, which is the most used excipient in compounding SR capsules.
Table 2.
Percentages of drug release from two different strengths prepared by the Alligation method.
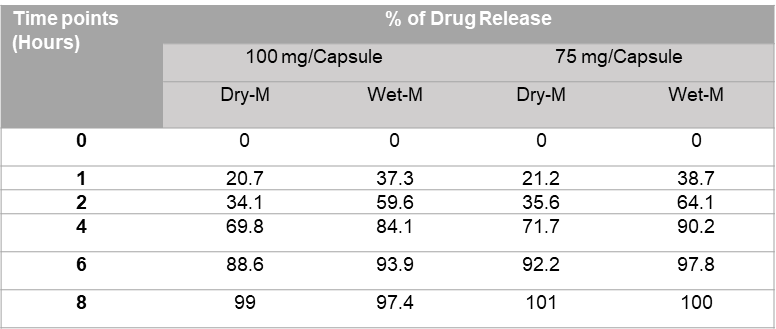
The results of the initial study have been reported in Table 2. It shows that 100% of the drug has been released by 8 hours for both dry and wet methods. The percent of drug released from 75 mg and 100 mg capsules prepared by dry mix and wet granulation followed the sigmoid pattern. Initially (at 1 and 2-hour time points), the % drug release from the 75 mg and 100 mg capsules prepared by the wet granulation method were significantly higher than the dry mix method. 50% of the thiamine was released in 2 hours from the SR capsule prepared by wet granulation process, whereas it took 3 hours from the SR capsule prepared by dry granulation process. However, the release pattern of thiamine HCl is more consistent throughout the 8 hours for the wet process in comparison to the dry method. If an SR capsule compounded with 40% Methocel E4M, based on the physicochemical properties of the drug, approximately 15% to 40% of the drug could be released in 1 hour, 30% to 70% of the drug could release in 2 hours, and more than 85% drug could release in 6 hours (Table 2 and Figure 2a).
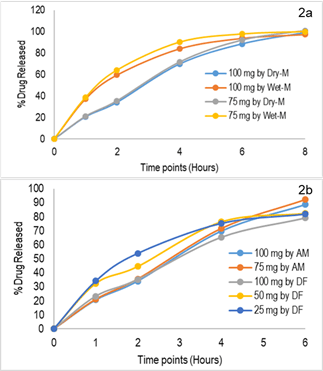
Fig. 2: 2a: Comparison of release kinetics for dry and wet granulation methods; 2b: Comparison of release kinetics SR capsules prepared by alligation method (AM) and direct filling (DF)technique. (Dissolution Medium: Water, 900 mL, Apparatus 2, 50 rpm)
The results of the second study are presented in Figure 2b. As previously mentioned, three different strengths (100%, 50%, 25%) of slow-release (SR) capsules were prepared by directly filling them into various capsule shells. It was observed that the percentage of drug release from the 100 mg strength capsules closely resembled the previously reported results (Fig. 2a). The 50 mg SR capsules exhibited slightly higher drug release, with approximately 10% more drug released within 2 hours compared to the 100 mg and 75 mg strengths capsules. For the lower strength SR capsules, there was a significant increase (>20%) in the percentage of drug release (Fig. 2b). This could be attributed to the smaller size of the capsule and the total fill weight/volume. Because matrices with lower polymer content or lower fill weight (smaller gel mass) do not exhibit proper slow release kinetics. Therefore, a minimum threshold of fill weight is necessary to ensure consistent drug release.
These findings suggest that different strengths such as 100%, 75%, 50%, and 25% of the drug-excipient powder matrix with specific release kinetics could be used for compounding SR capsules using the direct filling method. However, this approach may require additional product development costs, although it would reduce the workload of compounders. It is important to note that one limitation of this approach is that it may not always be possible to achieve a specific strength of the capsule due to the fixed fill volume of each capsule size. Also, there will be some variations in the drug release kinetics due to the volume effect. For example, the fill volume of the capsule shells can vary by 25%. If the fill weight of the 100 mg TH SR capsule is 425 mg in a 00-size capsule, the compounder would be able to fill approximately 319 mg in a 0-size capsule to make a 75 mg SR capsule. It’s not feasible to fill more and less than this filling capacity.
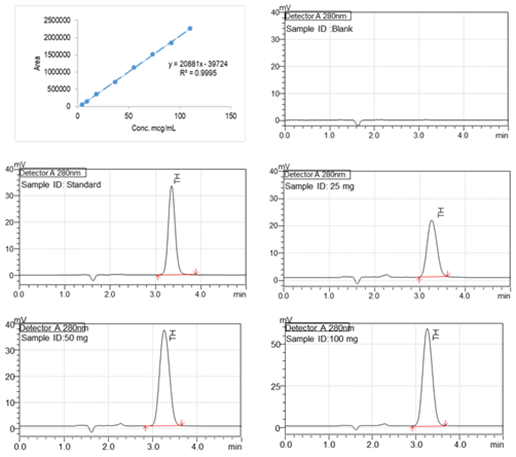
Fig. 3. Linear curve (5 µg/mL to 120 µg/mL) of lidocaine, Representative high-performance liquid chromatogram of the Diluent, TH Standard, and Test samples (SR Capsules at 2 hours time point). RT of TH is 3.2 minutes.
Section 503A and section 503B of the Federal Food, Drug, and Cosmetic Act applies to human drug compounding 10. 503B outsourcing facility to compound large quantities with proper quality control and assurance. The 503B pharmacy could develop different strengths (low and high strengths) of the drug excipient powder matrix for SR capsules with specific release kinetics. The 503B outsourcing facilities can conduct laboratory testing to ensure the consistency of the drug release for a specific period. The drug excipient powder matrix for SR capsules could be distributed to smaller pharmacies to compound SR preparations. 503A pharmacies could use the simple allegation calculation method to compound any strength or they can fill the premade blends directly to compound SR capsules as per the 503B outsourcing facilities specification. The excipient matrix could either be filled in capsule shells or compressed in a tablet press to create a tablet with better release kinetics. In the last twenty years, errors involving compounded preparations have brought awareness to the fact that without proper quality assurance, there is a lot of room for errors.11 We believe that this new idea will ensure the quality of the compounded SR capsule preparations.
Conclusions
The results of the SR compounded capsules from this study confirm our hypothesis. In conclusion, we suggest providing different premix blends distributed by a 503B outsourcing facility as per the US FDA regulations to 503A pharmacies to compound SR capsules. This approach will ensure appropriate prescriber dosing, adequate release profiles, and quality for sufficient efficacy and patient safety.
Acknowledgments
This research was funded by the Department of Pharmaceutical Sciences Faculty Research Budget at Appalachian College of Pharmacy, Oakwood, VA.
Conflict of interest
The authors declare no Conflict of interest.
References
- United States Pharmacopeial Convention, Inc. USP General Chapter 〈1151〉 Pharmaceutical dosage forms. [Link]
- Ullmann P. Practice update: Excipient selection for compounded pharmaceutical capsules: They’re only fillers, right? AJP Aust J Pharm. Published online August 1, 2017. Accessed January 5, 2024. [Link]
- APA Dictionary of Psychology. Accessed January 5, 2024. [Link]
- Zur E. Compounding slow-release capsules: a comprehensive review and an Excel spreadsheet for faster calculations of excipients. Int J Pharm Compd. 2013;17(1):10-22. [PubMed]
- Bakhteyar H, Cassone C, Kohan HG, Sani SN. Kinetic Analysis of Drug Release from Compounded Slow-release Capsules of Liothyronine Sodium (T3). Int J Pharm Compd. 2017;21(5):418-425. [PubMed]
- Bogner RH, Szweijkowski J, Houston A. Release of morphine sulfate from compounded slow-release capsules: the effect of formulation on release. Int J Pharm Compd. 2001;5(5):401-405. [PubMed]
- Glowiak DL, Green JL, Bowman BJ. In vitro evaluation of extemporaneously compounded slow-release capsules containing morphine sulfate or oxycodone hydrochloride. Int J Pharm Compd. 2005;9(2):157-164. [PubMed]
- Radojkovic B, Milić J, Calija B. Compounding of slow-release niacinamide capsules: feasibility and characterization. Int J Pharm Compd. 2012;16(5):434-437. [PubMed]
- Pinheiro VA, Kaneko TM, Velasco MVR, Consiglieri VO. Development and in vitro evaluation of extended-release theophylline matrix capsules. Rev Bras Ciênc Farm. 2007;43(2). [Link]
- Research C for DE and. Human Drug Compounding. FDA. Published December 12, 2023. Accessed January 3, 2024. [Website]
- Hall E, Levesque D, Rashid M, Mullins R, Hossain MF. A Simplified Method for Compounding Lidocaine Topical Gel: A Demonstration of Quality Assurance to Prevent Errors in 503A Pharmacies. Int J Pharm Compd. 2021;25(3):197-204. [PubMed]
Steps to be taken to correctly compound slow-release capsules: A Simplified Compounding Approach by Md. Mazharul Islam Chowdhury, Kenna Fields, Machaela Keene, Randy Mullins, Mohammad Faisal Hossain is licensed under CC BY 4.0
Note
Copyright © 2024 Chowdhury et al. Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.
