Abstract
Patients who are inappropriately or inaccurately labeled with a penicillin allergy are often limited to subpar second or third-line antibiotic therapy resulting in an increased risk of drug failure, hospital-acquired infections, and increased length of stay with a corresponding financial burden to match. Approximately 7-8% of individuals seeking healthcare in the USA have a documented penicillin allergy, although recent studies have found that approximately ⅓ of patients with a documented penicillin allergy initially presented with cutaneous symptoms matching an IgE-mediated reaction. Our study utilized the PEN-FAST survey to determine if there is an opportunity for antimicrobial stewardship amongst the penicillin-allergic patient population at Pikeville Medical Center. We found that 63% of our penicillin-allergic patient population obtained a PEN-FAST composite score of 0, 1, or 2, and are therefore strong candidates for de-labeling due to the low risk of less than a 5% chance of incurring a penicillin hypersensitivity type reaction. Based on our findings, de-labeling protocols may reach approximately ⅔ of the penicillin-allergic patient population, presenting a promising opportunity for institutions to improve their standardized antimicrobial administration ratio (SAAR) score.
Key Words: Beta-lactam Allergy De-labeling, Beta-lactam Hypersensitivity, Penicillin Allergy De-labeling, Penicillin Hypersensitivity, Antibiotic Stewardship
Introduction
Beta-lactams are a broad class of antibiotics that include penicillins, cephalosporins, carbapenems, and monobactams. Their versatility, substantial efficacy, safety, and cost-effectiveness make beta-lactams the most frequently prescribed antibiotic class, and as a result, the class for which hypersensitivity reactions are most often reported.1 Penicillin stands out within its class as not only the most prevalent beta-lactam allergy but also the most frequently reported drug class allergy, affecting approximately 7-8% of individuals seeking healthcare in the USA.2,3 Research indicates that 75% of individuals labeled with a penicillin allergy acquire this designation by the age of 3, with only ⅓ of those labeled presenting with a rash clinically consistent with an immunoglobulin E (IgE) – mediated hypersensitivity.4,7 Symptoms clinically significant for an IgE-mediated rash include urticaria with or without pruritus and erythema multiforme.3 Studies seem to reaffirm this metric with one reviewing 256 rashes associated with penicillins, and concluding that only 39.1% of rashes were thought to be IgE mediated and allergic in nature.3 Another looked at 85 children with documented penicillin allergy where only 31.7% of reactions were found to be classically IgE mediated.5 Furthermore, IgE-mediated penicillin allergy wanes over time with 80% of patients becoming tolerant after a decade, leading to a total risk of less than 5% for all documented penicillin-allergic patients to experience a genuine clinically significant IgE-mediated or T-lymphocyte mediated penicillin hypersensitivity reaction.6
These issues present a profound opportunity for antimicrobial stewardship as patients who are inappropriately or inaccurately labeled with a penicillin allergy are often limited to subpar second or third-line antibiotic therapy resulting in an increased risk of drug failure, hospital-acquired infections, and increased length of stay with a corresponding financial burden to match.1 The beneficial impact of penicillin de-labeling protocols on public and population health can not be overstated. Patients with a documented penicillin allergy average 10% more total hospital days, and are treated with significantly more fluoroquinolones, clindamycin, and vancomycin. This results in 23.4% more clostridium difficile (C. diff), 14.1% more methicillin-resistant staphylococcus aureus (MRSA), and 30.1% more vancomycin-resistant enterococcus (VRE) infections when compared to control subjects without a documented penicillin allergy.8 This directly translates to an increased financial burden, as patients with a documented penicillin allergy are found to have an additional $609/patient of direct drug costs incurred during inpatient admission.9 Additionally, outpatient prescription costs are estimated to be $14 to $193/patient higher for penicillin-allergic patients.9 Patients without a documented penicillin allergy incur less inpatient costs with average savings ranging from $1,145 to $4,254/patient.9
The implementation of penicillin allergy de-labeling protocols provides an opportunity to remove the undue burden an inaccurate penicillin allergy label confers on patient finances, outcomes, and health. Our study aims to quantify the percentage of our penicillin-allergic patient population fit for de-labeling according to the parameters set forth by the PEN-FAST survey. The PEN-FAST survey has been validated to identify low-risk penicillin allergies and has been endorsed by the Kentucky Antimicrobial Stewardship Innovation Consortium (KASIC).10
Methods
PEN-FAST, a clinical tool used to quantify the risk of penicillin allergies, was used for data collection. This tool was found to be simple and accurate at identifying low-risk penicillin allergies, which enabled the opportunity to delabel without formal allergy testing.11 The exclusion of allergy testing is stratified by a negative predictive value of 96.3% with a composite score of less than 3 on the PEN-FAST assessment.12 In Kentucky, inpatient antimicrobial use exceeds national rates. KASIC utilizes PEN-FAST as the primary tool for de-labeling and antimicrobial stewardship guidelines.13 In 2021, the World Health Organization published an antimicrobial stewardship guideline. The use of history-based allergy assessments, such as PEN-FAST, is an effective strategy to promote antimicrobial stewardship.14
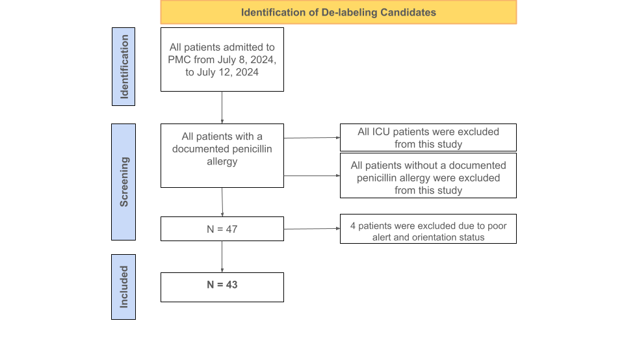
Figure 1: Flow chart of the identification of the de-labeling candidates.
The main features of this clinical trial include utilizing PEN-FAST for direct patient surveying and correlating patient responses to validated risk categories. The inclusion criteria for this survey was any patient with a positive penicillin allergy label on the electronic health record at Pikeville Medical Center; a 348-bed acute care facility located in Pikeville, Kentucky. The exclusion criteria included (i) patients without a positive penicillin allergy label and (ii) all ICU patients. The survey was conducted over a 5-day period and included 47 patients in total. All participants were assessed on the criteria of PEN-FAST. The features of this clinical validation tool include confirmation of a penicillin allergy from the patient or immediate relative, length of time since reaction (5 years or less), type of reaction (anaphylaxis/angioedema or severe cutaneous reaction), and treatment, if any, required for the penicillin reaction. PEN-FAST utilizes numerical scores to categorize patients as very low risk, low risk, moderate risk, or high risk. If a patient reports a reaction that occurred in five years or less, then 2 points are given. If the patient reports anaphylaxis, angioedema, or severe cutaneous reactions, such as urticaria or Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN), then 2 points are given. If treatment was required for a reaction, such as over-the-counter Benadryl or hospitalization, then 1 point is given.11
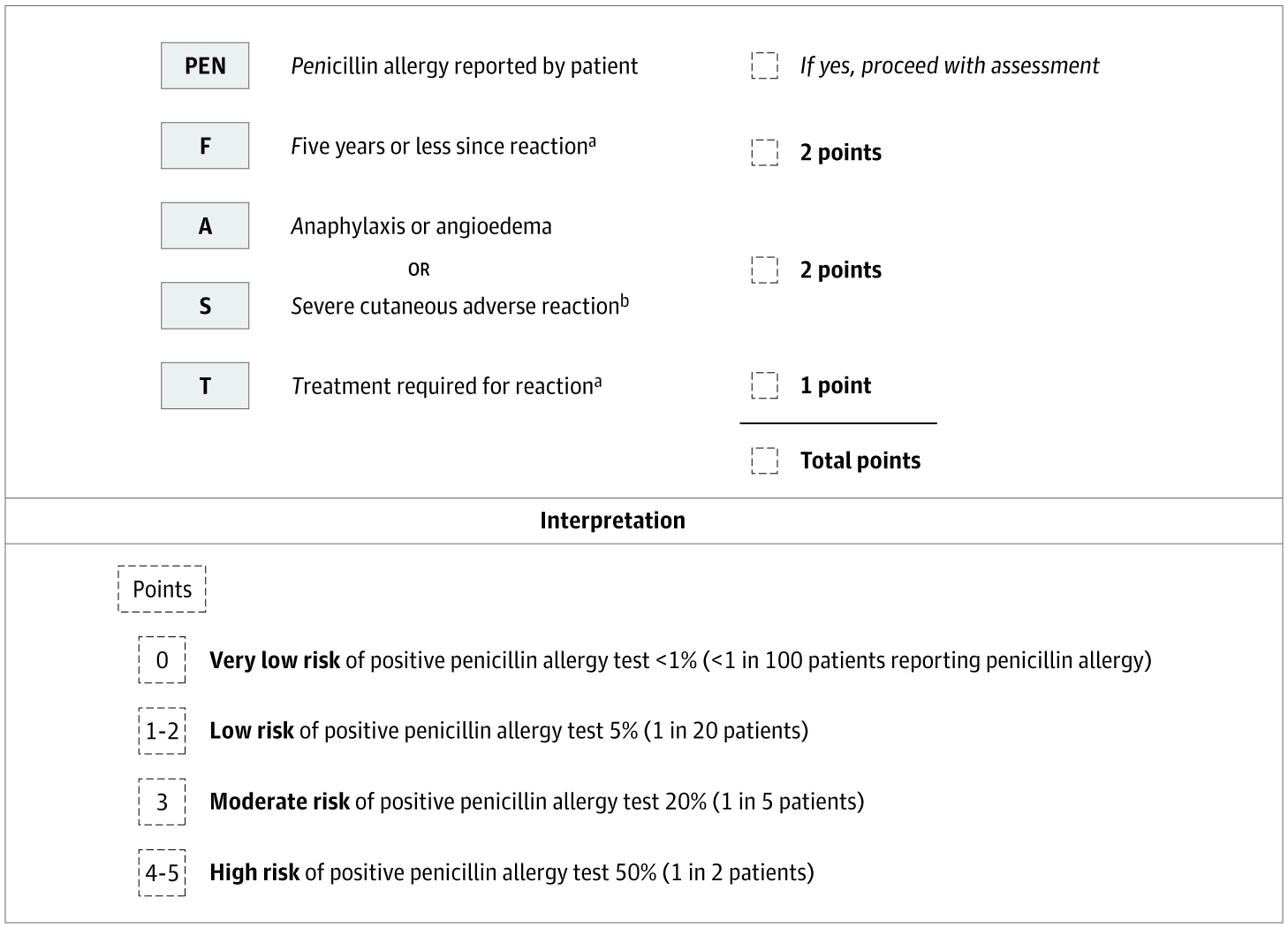
Figure 2: PEN-FAST Penicillin Allergy Clinical Decision Rule [Figure 2 has been adapted with permission from the corresponding author Dr. Trubiano. We sincerely appreciate their generosity in allowing us to utilize this figure for our research.]
A patient with a composite score of 0 is deemed very low risk with a less than one percent chance a penicillin reaction will occur. A score of 1-2 is low risk with a less than five percent chance for a penicillin reaction. A score of 3 is moderate risk, and a score of 4-5 is high risk with a fifty percent chance for a penicillin reaction.11 All patients with a total PEN-FAST score of 0-2 will be de-labeled. The 47 non-ICU patients included in this study were questioned and evaluated by student pharmacists. Of the 47 surveyed, 4 patients were not alert and oriented, which resulted in non-applicable responses. These responses were still recorded and included separately in the final data. Of the remaining 43 patients, around 60% had a composite score between 0-2 and were eligible for de-labeling. The de-labeling process includes termination of the penicillin allergy on each patient’s electronic health record and patient education from a pharmacist. The main goals of patient education were informing patients of the removal of the penicillin allergy and the benefits/importance of removing an insignificant allergy from their health records.
Results
Of the 43 patients we surveyed from July 8th, 2024 to July 12th, 2024, 27 patients (63%) received a PEN-FAST score of 0, 1, or 2, and were therefore considered strong candidates for de-labeling due to the minor risk of less than a 5% chance of incurring a penicillin hypersensitivity type reaction. Conversely, 37% of our sample size received a PEN-FAST score of 3, 4, or 5, identifying these patients as moderate to high risk for incurring an IgE-mediated penicillin allergic reaction. Our findings match those of other studies that identified approximately ⅓ of all documented penicillin-allergic patients as moderate to high risk for an IgE-mediated reaction.3,4,5,7
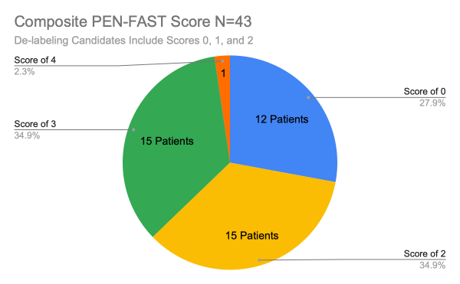
Figure 3: Survey Results Composite PEN-FAST Score.
Our results extrapolated for individual PEN-FAST scoring components offer additional insights. Out of 43 patients, only 2 (5%) reported a reaction within the past 5 years, while the remaining 41 patients (95%) reported more than 5 years since their last penicillin allergic reaction. This finding further outlines the potential scope of penicillin de-labeling protocols as IgE-mediated penicillin allergy wanes over time, with 80% of patients becoming tolerant after a decade.6 Thirteen (30%) patients surveyed reported the absence of anaphylaxis/angioedema or a severe cutaneous adverse reaction while the remaining 30 (70%) reported having experienced 1 or more of these symptoms. Of the 43 patients surveyed, 15 (35%) reported having received treatment for their reaction, with the remaining 28 (65%) patients not requiring or forgoing treatment for their reaction.
Discussion
In Kentucky, inpatient antimicrobial use far exceeds national utilization.13 This discrepancy is measured using the standardized antimicrobial usage ratio (SAAR), a metric that compares observed institutional antimicrobial use to the predicted use of similar sized facilities; allocating a score of 1 if antimicrobial use is equivalent to predicted use, a score of greater than 1 if antibiotics are being overutilized, and a score of less than 1 if antibiotics are being underutilized.15,16 In 2022, the Kentucky median SAAR value for broad-spectrum antibacterial agents predominantly used for hospital-onset infections (BSHO) of 1.127 was found to be significantly higher than the respective national average of 1.019, outlining the need for additional antimicrobial stewardship initiatives within the state.13 SAAR scores by institution may identify even greater disparities between the national, state, and local levels. The institutional BSHO SAAR score recorded from January to June of 1.55 at Pikeville Medical Center substantiates this discrepancy.
Penicillin de-labeling initiatives should not be overlooked as a viable tool in addressing the discrepancy between national, state, and local SAAR scores. De-labeling protocols have the capacity to reach 7-8% of the entire patient population, with approximately ⅔ of the penicillin allergic population potentially fit for de-labeling according to our study’s PEN-FAST survey results, and the prevalence of IgE-mediated allergic reactions amongst penicillin-allergic patient populations.2,3,4,5,7
Recommendation
The PEN-FAST survey tool effectively identifies individuals who are at low risk of experiencing a penicillin allergy as potential candidates for de-labeling.10,11 For those patients who received a score of 3, 4, or 5 and are considered moderate to high risk for experiencing a penicillin-allergic reaction, confirmation of penicillin allergy diagnosis or de-labeling using direct oral drug challenges or penicillin skin testing seems to be safe and is associated with a low rate of adverse reactions.17 Pikeville Medical Center is currently implementing a penicillin allergy de-labeling desensitization protocol to facilitate the re-evaluation of all documented penicillin allergy labels through either direct oral drug challenges or penicillin skin testing amongst all penicillin-allergic patients. We recommend institutions implement similar follow-ups to their de-labeling protocols to ensure accurate documentation of all penicillin allergy labels within their respective institutions.
Conclusion
Beta-lactam antimicrobials are frequently prescribed and utilized in both inpatient and outpatient settings. Beta-lactams, specifically penicillin, have the highest prevalence of reported hypersensitivity reactions. Unfortunately, a true IgE-mediated allergy is rare and only quantifiable in a slim percentage of the penicillin-allergic population. This can lead to clinically irrelevant penicillin reactions reported in patient profiles. The occurrence of inaccurate allergy reporting can correlate to high utilization of broad-spectrum antimicrobials and the development of multidrug-resistant infections, resulting in discrepancies in SAAR scores. In this clinical study, 63% of patients surveyed were eligible for de-labeling based on the PEN-FAST criteria. Despite a small study population of 47 patients, the data demonstrates the high prevalence of insignificant penicillin allergy labels at Pikeville Medical Center. Penicillin de-labeling protocols may reach 7-8% of individuals seeking healthcare in the USA, identifying approximately two-thirds of those with a documented penicillin allergy as candidates for low-risk de-labeling. By enabling antimicrobial initiatives, improvement of institutional antimicrobial utilization and SAAR scores will be evident. In the future, SAAR scores and appropriate antimicrobial selection may be used for reimbursement. This further necessitates the need for institutions to partake in de-labeling protocols.
Acknowledgments
This research project was completed during the Advanced Pharmacy Practice Experience Block 1 rotation at Pikeville Medical Center with preceptor Dr. Cydney McCoy, Clinical Pharmacist. The authors would like to issue a special thanks to Cydney McCoy, PharmD, Yuliya Blackburn, PharmD, and Ashley Holland, PharmD, as well as the Pikeville Medical Center Inpatient pharmacy department as a whole for their guidance and direction. We would also like to thank Saraf Amin and Brittany Russell, PharmD Candidates 2026 at the Appalachian College of Pharmacy, for their assistance in data collection.
Authors’ Contributions
Each author actively engaged in analyzing and discussing the results, thereby contributing to the finalization of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Powell N, Stephens J, Kohl D, et al. The effectiveness of interventions that support penicillin allergy assessment and delabeling of adult and pediatric patients by nonallergy specialists: a systematic review and meta-analysis. International Journal of Infectious Diseases. 2022;129(129):152-161. doi:https://doi.org/10.1016/j.ijid.2022.11.026
- Macy E. Penicillin and Beta-Lactam Allergy: Epidemiology and Diagnosis. Current Allergy and Asthma Reports. 2014;14(11). doi:https://doi.org/10.1007/s11882-014-0476-y
- Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic Rashes in Children. Archives of Dermatology. 2000;136(7). doi:https://doi.org/10.1001/archderm.136.7.849
- Ana Maria Copaescu, Vogrin S, James F, et al. Efficacy of a Clinical Decision Rule to Enable Direct Oral Challenge in Patients With Low-Risk Penicillin Allergy: The PALACE Randomized Clinical Trial. JAMA Internal Medicine. 2023;183. doi:https://doi.org/10.1001/jamainternmed.2023.2986
- Pichichero ME, Pichichero DM. Diagnosis of penicillin, amoxicillin, and cephalosporin allergy: Reliability of examination assessed by skin testing and oral challenge. The Journal of Pediatrics. 1998;132(1):137-143. doi:https://doi.org/10.1016/s0022-3476(98)70499-8
- Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and Management of Penicillin Allergy. JAMA. 2019;321(2):188. doi:https://doi.org/10.1001/jama.2018.19283
- Vyles D, Chiu A, Simpson P, Nimmer M, Adams J, Brousseau DC. Parent-Reported Penicillin Allergy Symptoms in the Pediatric Emergency Department. Academic Pediatrics. 2017;17(3):251-255. doi:https://doi.org/10.1016/j.acap.2016.11.004
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. Journal of Allergy and Clinical Immunology. 2014;133(3):790-796. doi:https://doi.org/10.1016/j.jaci.2013.09.021
- Mattingly TJ, Fulton A, Lumish RA, et al. The Cost of Self-Reported Penicillin Allergy: A Systematic Review. The Journal of Allergy and Clinical Immunology: In Practice. 2018;6(5):1649-1654.e4. doi:https://doi.org/10.1016/j.jaip.2017.12.033
- Piotin A, Godet J, Trubiano JA, et al. Predictive factors of amoxicillin immediate hypersensitivity and validation of PEN-FAST clinical decision rule. Annals of Allergy, Asthma & Immunology. 2022;128(1):27-32. doi:https://doi.org/10.1016/j.anai.2021.07.005
- Trubiano JA, Vogrin S, Chua KYL, et al. Development and Validation of a Penicillin Allergy Clinical Decision Rule. JAMA internal medicine. 2020;180(5). doi:https://doi.org/10.1001/jamainternmed.2020.0403
- Copaescu AM, James F, Vogrin S, et al. Use of a penicillin allergy clinical decision rule to enable direct oral penicillin provocation: an international multicentre randomised control trial in an adult population (PALACE): study protocol. BMJ Open. 2022;12(8):e063784. doi:https://doi.org/10.1136/bmjopen-2022-063784
- Song M, Wilde AM, Song CM, et al. Kentucky Antimicrobial Stewardship Innovation Consortium (KASIC): A Program to Improve Antibiotic Use in the Commonwealth. Norton Healthcare medical journal /Norton Healthcare medical journal. 2023;1(2). doi:https://doi.org/10.59541/001c.83286
- WHO. Antimicrobial stewardship interventions: a practical guide. www.who.int. Published April 12, 2021. https://www.who.int/europe/publications/i/item/9789289056267
- Butterfield-Cowper J. Best Practice Proposal to Enhance Application of the Standardized Antimicrobial Administration Ratio (SAAR). HCA Healthcare Journal of Medicine. 2023;4(2). doi:https://doi.org/10.36518/2689-0216.1560
- Avedissian SN, Rhodes NJ, Liu J, et al. Understanding the Components, Calculation, and Impact of Monthly and Seasonal Variation of the Standardized Antimicrobial Utilization Ratio (SAAR). Antimicrobial agents and chemotherapy. 2019;63(3). doi:https://doi.org/10.1128/aac.01780-18
- Rui Providencia, Ghazaleh Aali, Zhu F, et al. Penicillin Allergy Testing and Delabeling for Patients Who Are Prescribed Penicillin: A Systematic Review for a World Health Organization Guideline. Clinical Reviews in Allergy & Immunology. 2024;66. doi:https://doi.org/10.1007/s12016-024-08988-2
Penicillin Allergy De-labeling: A Missed Opportunity for Antimicrobial Stewardship © 2024 by Shobhit Sood and Emily Lester is licensed under CC BY 4.0
Note
Copyright © 2024 Sood S and Lester E. Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.
