Abstract
In the field of pharmacy, compounding is a practice that allows for a more customizable drug formulation. A more recent improvement to the compounding process has been the creation of the autocompounder. This study was conducted by the PharmD students as part of their APPE research elective project to evaluate the performance of automatic compounding compared to manual compounding. To demonstrate this, troches were manually compounded and created with an automatic compounder. They were then tested for drug content uniformity with the HPLC, and checked for weight variation. The average weight for all manually compounded troches (96.2% ± 2.4) and the automatically compounded troches (102.9% ± 0.9) were within the specifications. With the current trend of technological advancement, the autocompounder is likely to become irreplaceable in the next few years. The most significant advantage of using the autocompounder is compounding different strengths from the same mixture without compromising the dose accuracy and product quality. Another significant advantage is the potential relief from stress and workload for pharmacy professionals due to automation. Cartridges prefilled with drug mixtures can significantly and positively impact delivery time, product cost, quality of product, and the compounder. At present, autocompounders may be more appropriate for 503B pharmacies or larger 503A pharmacies.
Keywords: Autocompounder, Future Prospect, 503A Pharmacy, Compounding Nonsterile Preparations, Product Quality.
Introduction
Pharmaceutical compounding, as defined by the U.S. FDA, is generally “a practice in which a licensed pharmacist, a licensed physician, or, in the case of an outsourcing facility, a person under the supervision of a licensed pharmacist, combines, mixes, or alters ingredients of a drug to create a medication tailored to the needs of an individual patient”.1 The future of patient care is trending towards individualized healthcare. This has obvious effects within the pharmaceutical realm, as it is now common practice for 503A compounding pharmacies to create customized dosage forms for patients as requested by their healthcare providers. This will expand as the healthcare industry gears towards pharmacogenomics, which uses genomic information for more specialized personal drugs.2 This upward trend in patient-centric drug formulations will necessitate more accurate and streamlined compounding processes.
Automation in compounding could be a solution to this need through the efficient production of quality customizable medications. Currently, 503A pharmacies are not regulated like manufacturers or outsourcing facilities. The drugs are compounded either by a licensed pharmacist by hand or under the supervision of a licensed pharmacist, leaving room for human error.3 According to an extensive case report from Pew Charitable Trust, there have been 1,416 reported cases and 114 reported deaths in the United States from 2001-2017 due to issues with compounded medications.4 Most of the cases were caused by contamination or dosing errors that occurred while compounding the drugs.5
Autocompounders have traditionally been used in hospital pharmacies to compound sterile formulations such as Total Parenteral Nutrition (TPN) formulations or IV bags. This has allowed for quick individualization of medication for patients and has reduced the probability of adverse reactions due to contamination or dosing errors. A systematic review suggests the positive impacts of automation of in-hospital pharmacy dispensing to improve the reliability and efficiency of the medication process.6 Like automatic compounders used in sterile preparation, a non-sterile autocompounding machine can streamline time-consuming and error-prone processes. By implementing the use of autocompounders in a 503A pharmacy setting, pharmacists can worry less about having to compound drugs by hand and feel assured they are guaranteeing the patient a drug product that is individualized and efficiently made for them. An autocompounder, essentially used for non-sterile compounding, is a machine designed to accurately dispense the desired dose of a compounded drug formulation into a mold. Automation in compounding through a variety of dosage forms is possible to compound troches, gummies, capsules, sublingual, and buccal delivery.7 Since the autocompounder is relatively new in nonsterile compounding, our goal in this study was to compare the quality and performance of the compounded preparations made by auto compounder and manually.
Materials
USP-grade drug substances and excipients were used to compound diphenhydramine HCl troches, and ACS or high-performance liquid chromatographic (HPLC)-grade chemicals were used to analyze the formulation and evaluate the quality of the preparation. The following materials were purchased from the commercial suppliers/manufacturers: Diphenhydramine HCl (Medisca), Acetonitrile (EMD Millipore), Phosphoric acid (Alfa Aesar), Monobasic potassium phosphate (VWR), Polyethylene Glycol 1450 (Medisca), Acacia (PCCA), Silica Gel (PCCA), and Stevia (Medisca). Distilled water was obtained through the in-house water distillation system (model SZ-93A, China).
Methods
Troches are a type of soft lozenge that can be either slowly dissolved or chewed by the patient. These lozenges contain a base, such as PEG 1450, that gives it a soft texture.8,9 Some formulations may contain acacia, silica gel, and sweetening agents. Acacia and silica gel are used as a suspending agent and add texture and smoothness.10 For this study, three batches of troches were made using the auto compounder and three manually. A simple formulation and compounding method were used to reduce errors and compare the quality of manual and automatic compounding. The batches from the manually compounded and automatically compounded batches were from the same initial mixture, and this minimized the error of contained uniformity. The mixture was split between the manual and auto compounder when pouring into the mold and cartridge. The complete compounding process and master formula are reported in Table 1. The performance was evaluated by the time it took to make the product, the uniformity of the product, and the quality of the product.
Table 1
The master formula for 100 troches. Each Troche contains 10 mg of diphenhydramine hydrochloride and weighs 1 g.

Compounding Direction and Procedure:
- First, weigh the PEG 1450 into a glass beaker and melted on a hot plate at 60°C.
- Then, weigh and transfer the following ingredients (Diphenhydramine Hydrochloride, Acacia, Silica Gel, and Stevia) into a glass mortar and triturate for 3 minutes.
- Next, mix the triturated powder into the melted PEG 1450 using a sieve and swirl for 2 minutes.
- Sonicate the suspension in hot water (50°C-60°C) for 3-5 minutes.
For manual compounding
Pour the mixture into a troche mold and weigh the troche mold after it cools.
For automatic compounding
- Pour the mixture into a cartridge.
- Remove the plunger from the auto compounder and calibrate the machine.
- Place the cartridge in the machine and allow it to warm, if necessary.
- Place the troche mold in the appropriate location.
- Prime the autocompounder (Vitae Industries, Inc7), and the machine fills the mold in the troche and waits until it hardened (takes about 10 minutes).
Quality Control: Visual inspection and check the weight of the troche.
Storage Condition: Store at a Controlled Room Temperature
Beyond-Use Date: The BUD is not later than 90 days.11
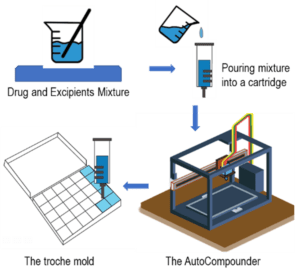
Fig. 1: The process flow of the AutoCompounder.
HPLC Analysis
The HPLC method (Mobile phase and Chromatographic conditions) used to determine the content of Diphenhydramine Hydrochloride (DPH) was adopted from the United States Pharmacopeia (USP) monographs. To determine the potency, the active ingredient (DPH) was extracted using purified water with 10 minutes of sonication and intermittent shaking. The content of DPH was determined using the standards curve (0.174 mg/mL to 0.260 mg/mL) of DPH (Figure 2B). The chromatographic separation was achieved with a Shimadzu Prominence HPLC system using isocratic elution mode with a flow rate of 1 mL/min on a Waters Xterra MS C-18 column equipped with an ultraviolet-visible (UV-Vis) detector (220 nm). The mobile phase of the reverse-phase HPLC was composed of acetonitrile and freshly made buffer (0.04M monobasic potassium phosphate; pH adjusted to 3.0 with phosphoric acid) in the ratio of 35:65 (v/v). FS60 Sonicator (Fisher Scientific, Massachusetts) was used during the extraction phase of the formulations. All weights were measured using a pre-calibrated analytical balance Mettler Toledo PB303-S/FACT (Mettler Toledo, Switzerland).
Results Discussion
For this study, the weights of the manually and automatically compounded troches were compared. The weight assessment results of the compounded troche preparations are reported in Table 2. The average for all manually compounded troches was 96.2% ± 2.4 and the average for the automatically compounded troches was 102.9% ± 0.9. The second manually composited samples had outliers, mainly due to improper filling of the troche mold. Validating formulas and compounding procedures, as well as creating master formulations and compounding records for each preparation, are essential. When using the automatic compounder, a new formulation may need to be developed for optimal drug content uniformity. In this study, aspartame was initially used as a sweetening agent in the formula. Several times, the opening of the cartridges was clogged due to aspartame dispersing the mixture unevenly. The formula was optimized to reduce the variability by replacing aspartame with saccharin. This resulted in visibly greater uniformity in the mixture with no visible aggregates. The results obtained by HPLC analysis for the automatically compounded troches was 103.3% ±1.04, whereas the manually compounded troches were 99.9% ± 0.6. Both automatic and manual compounding require personnel to receive appropriate training. Figure 2 shows the representative HPLC chromatograms and the standard linear curve.
Table 2
Quality Test Results (Weight Assessment) of the Compounded Troche Preparation.
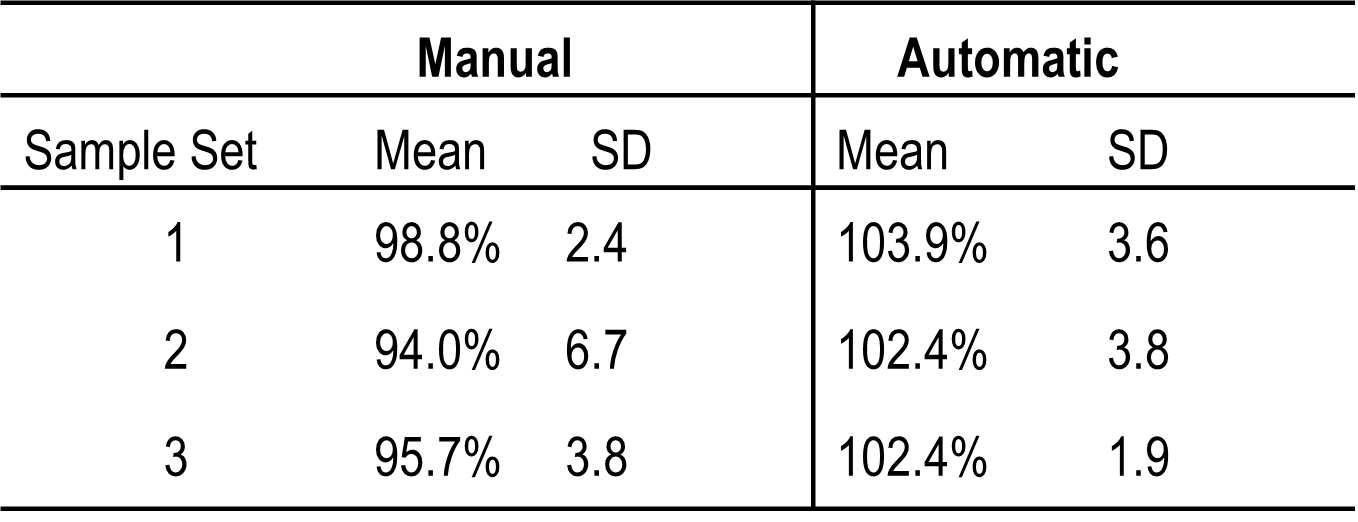
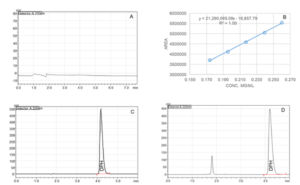
Fig. 2. Representative high-performance liquid chromatogram of the Blank (A), Standard (C) and Test sample (D). B: Linear curve (0.174 mg/mL to 0.260 mg/mL) of Diphenhydramine Hydrochloride (DPH).
The autocompounder will be the future of compounding. Currently, however, each pharmacy will need to evaluate the pros and cons of the autocompounder and decide if it is reasonable for them. The results suggest that there is no significant difference between the two methods. Considering research time, cost, and process loss, the authors feel that using an autocompounder may not be a good option for a small pharmacy that only compounds a few monthly prescriptions. But it can be a good option for big pharmacies as they can pre-fill some tubes and keep them in proper storage. Using pre-filled cartridges, the pharmacist can compound a product faster than if they were compounding manually. The only step in the process would be to heat the cartridge, which is significantly faster than weighing the ingredients and creating the mixture.
It has been shown with other examples of automation, such as an automated dispensing system at a National Health Service hospital in Wales, UK, that methods that minimize human error and save time reduce staff stress and workload.12 This improves working conditions and allows staff to have better morale and focus attention on other tasks that may require more diligence. The authors also believe that once the autocompounder becomes more widespread, drug manufacturers can also sell prefilled cartridges to reduce the workload on pharmacists and technicians further, thus improving efficiency and possibly saving costs on materials.
Conclusions
The field of pharmacy is moving towards patient-centric formulation and dosing. Though advances in compounding automation are integral to this paper, this may not be a cost-effective option for traditional pharmacies. Automated compounders may be better suited to the larger 503A pharmacies or 503B outsourcing facilities. With the current trend of technological advancement, the auto compounder may become irreplaceable in the next few years. Using prefilled cartridges can be a good option for traditional pharmacies to reduce costs and produce high-quality preparations. As new types of autocompounders develop with time, the possibilities and applications of this may lead to high-quality personalized drugs for patients.
Acknowledgments
The authors are thankful to Dr. Randy Mullins, Associate Professor of Pharmacy Practice and Department Chair of the Pharmaceutical Sciences Department, Dr. Alkhalil Housni, PharmD, ACP Alumni, and Shoshi Chowdhury, Student at Marshall University School of Medicine for their time and effort in reviewing the manuscript and providing inputs. The authors are also grateful to Dr. Md. Mazharul Islam Chowdhury, Postdoctoral Faculty in the Department of Pharmaceutical Sciences at Appalachian College of Pharmacy for drawing Figure 1.
References
- FDA. Human Drug Compounding. Accessed January 3, 2024. [Website]
- What is pharmacogenomics? Accessed January 4, 2024. [Website]
- FDA. FD&C Act Provisions that Apply to Human Drug Compounding. Accessed January 3, 2024. [Website]
- The Pew Charitable Trusts. S. Illnesses and Deaths Associated With Compounded Medications or Repackaged Medications, 2001-17. Accessed January 4, 2024. [Website]
- Watson CJ, Whitledge JD, Siani AM, Burns MM. Pharmaceutical Compounding: a History, Regulatory Overview, and Systematic Review of Compounding Errors. J Med Toxicol. 2021;17(2):197-217. [Crossref]
- Batson S, Herranz A, Rohrbach N, Canobbio M, Mitchell SA, Bonnabry P. Automation of in-hospital pharmacy dispensing: a systematic review. Eur J Hosp Pharm Sci Pract. 2021;28(2):58-64. [PubMed]
- Vitae Industries Inc. The AutoCompounder. Accessed January 3, 2024. [Website]
- Elder Deborah Lester. A Practical Guide to Contemporary Pharmacy Practice and Compounding. 4th Edition. Wolters Kluwer Health, Inc. 2017 [Website]
- Loyd V. Allen Jr. The Art, Science, and Technology of Pharmaceutical Compounding. 6th Edition. Chapter 14: Lozenges, Troches, and Films. The American Pharmacists Association; 2020. [Crossref]
- University of North Carolina. Formulation Records. Accessed January 4, 2024. [Website]
- United States Pharmacopeial Convention, Inc. General Chapter <795> Pharmaceutical Compounding – Nonsterile Preparations. Accessed January 3, 2024. [Website]
- James KL, Barlow D, Bithell A, et al. The impact of automation on pharmacy staff experience of workplace stressors. Int J Pharm Pract. 2013;21(2):105-116. [Crossref]
Prospects and Challenges of Using an Autocompounder in a 503A Pharmacy for Non-Sterile Compounding: A Comparative Study © 2024 by Mohammad Faisal Hossain, Makayla Funk, Aaron Ratliff, Dalton Oliver is licensed under CC BY 4.0 ![]()
Note
Copyright © 2024 Hossain et al. Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.
